Slime is the world’s most popular chemistry demo, but most leave the table thinking it’s just magic goo. Let's change that!
What makes slime stretchy?
Slime is a material system. Its stretchiness arises from the formation of polymer chains into a network.
If you're interested, here's the chemistry of slime:
Polyvinyl alcohol (PVA) is a long-chain polymer. In a bottle of school glue, these chains slide past each other easily, which is why glue is a liquid.
When you add a borax solution (sodium tetraborate), it dissolves to create borate ions.
A single borate ion can form hydrogen bonds with the hydroxyl groups on two different PVA strands simultaneously.
This links the independent strands together into a three-dimensional network. This process is called cross-linking.
Instead of sliding past each other, the strands are now tethered. This transforms the liquid glue into a non-Newtonian fluid (slime) that retains its shape yet still flows slowly.
Acetic acid is a weak acid that provides hydrogen ions to the mixture.
The borate ions form more stable bonds with these structures than they do when hanging onto the PVA strands.
The acid reacts with the borate ions to form boric acid.
Boric acid does not cross-link PVA nearly as effectively as borate ions do. As the borate ions are neutralized.
The PVA strands are released from their network and revert to independent chains, restoring the slime to a liquid state.
In this project, we aim to make slime, not just a demonstration, but a genuine exploration into the materials science of slime.
Building the game
The goal is to build a 3D slime chemistry game that allows players to experience the answer to our anchoring question: “What makes slime stretchy?”
Here’s our plan:
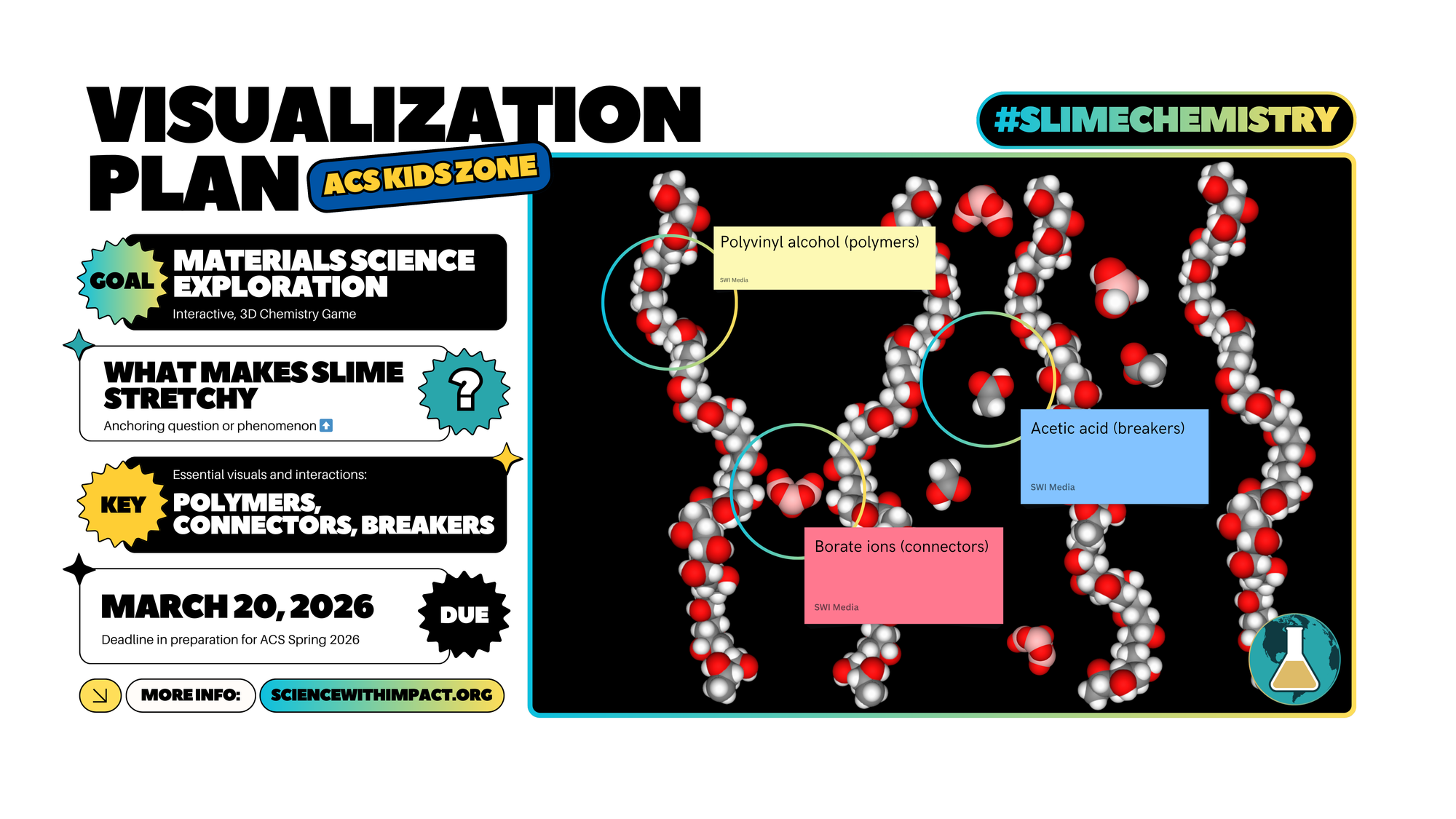
- Connectors represent the chemistry that increases cross-linking, so the material becomes more elastic and holds together.
- Bond breakers represent the chemistry that reduces cross-linking, so the material flows more like a liquid again.
That turns slime from a demonstration into a real exploration of material behavior.
But why a game?
We’ve already tested and provided other tangible experiences for our learners: making the slime by experimenting with different ratios and chemical connectors and bond breakers, and exploring the chemistry through beads and molecular models:

We even created a 3D molecular animation of the chemistry of slime:
But a game rewards curiosity by making the anchoring question answerable through play-based hypothesis testing: try, observe, change one variable, try again.
“Slime is already fun. The game makes it explainable.” - Vanessa Rosa, Ph.D.
Become a Member! (FREE)
1️⃣ Suggest what to build next.
2️⃣ Learn STEM storytelling.
3️⃣ Play early simulators.



Member discussion